Clinical Evaluations
ALL-IN-ONE FEDERAL OSHA/GHS & HIPAA/Omnibus Rules Training & Compliance Package
Consultants’ Comments
- “I thought our office was compliant until this program made me aware of what I didn’t know.”
- “ALL-IN-ONE FEDERAL OSHA & HIPAA Training & Compliance Package takes an overwhelming subject and makes it manageable.”
- “It was very comprehensive. So much more intensive than any other OSHA or HIPAA training I have ever had. It was really great.”
- “We have signed up for the yearly updates.”
- “Webinar was fast paced. A lot of information to digest.”
- “There is some redundancy in the paperwork.”
- “Separate the webinar into two sessions, one for OSHA and one for HIPAA.”
Description
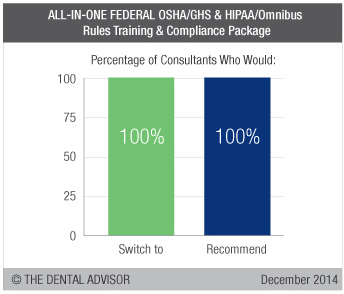
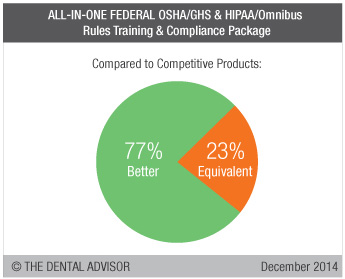
The ALL-IN-ONE FEDERAL OSHA/GHS & HIPAA/Omnibus Rules Training & Compliance Package is a comprehensive program providing everything necessary to comply with Annual OSHA Employee Training & Certification, Global Harmonization System (GHS) requirements and HIPAA Omnibus Rule compliance. Included are newly updated OSHA & HIPAA Manuals; a training CD-ROM that provides a 45-minute GHS & HIPAA employee training videos plus all required electronic forms; Biomedical Waste & Hazardous Rating labels; two pictogram diagram; federal OSHA poster; and a 72-Point OSHA Safety Facility Report with safety recommendations instantly emailed to your dental office. Training includes a 90-minute interactive webinar for the entire team to complete required training and a 90-minute phone consultation with the office’s key contact person to navigate through all materials. Necessary paperwork is organized in quick-reference binders. In case of an OSHA inspection, experts will be available to guide dentists through the process. One year of on-call support is included. Annual OSHA Employee Training & Certification renewal with program updates is available for a minimal cost. ALL-IN-ONE FEDERAL OSHA/GHS & HIPAA/Omnibus Rules Training & Compliance Package was evaluated by 13 consultants. This compliance program earned a 98% clinical rating.
Observations
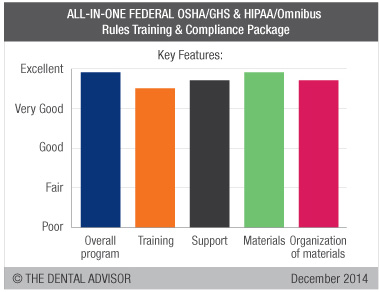
Overall Program
Dental Enhancements provided a concise, organized program that was customized for each dental office. OSHA and HIPAA rules are complex and change frequently, and this program not only tells offices what to do, but provides the materials and protocol checklists to ensure compliance with the federal regulations. Consultants reported that they were left with a sense of well-being that they were compliant and prepared in the event of an inspection.
Training
The 90-minute live webinar was presented to each dental office and served to fulfill all three Federal training requirements. An abundance of information was presented, which did not allow time for many questions or discussions. Rather, questions or clarifications were noted by the OSHA and HIPAA officers and resolved in private phone communications with Dental Enhancements.
Support
The initial 90-minute phone call with Dental Enhancements was flexible in terms of time and varied depending on each office’s needs. While 90 minutes was average, no limit was placed on this communication. Calls could be separated for offices that had different individuals who managed OSHA and HIPAA. Support was very methodical and individualized, walking dental personnel through the binders in order to complete forms and identify any needs unique to the dental practice. Follow-up calls were welcomed and were answered promptly.
Materials
Included in this compliance program are all forms and documents required to comply with OSHA/GHS and HIPAA regulations. Most useful were the office checklists for items necessary for compliance. The system even included adhesive labels for dental products and hazards. State-specific OSHA and HIPAA compliance was covered where required.
Organization of Materials
Materials were well organized in binders that kept required paperwork in one place. Four separate binders were included: OSHA Manual, Sterilization and Disinfection Log Organizer, SDS Organizer and HIPAA Manual. Each binder contained tabbed separators for quick access to the desired subject. Each section began with an outline summarizing the subject and the contents of the section.
Clinical Tips
- Be prepared to spend additional time updating MSDS forms to the new SDS standards of print and online availability.
- Use the included instructions for new Hazard Rating labeling requirements for dental products.
Editors’ Note:
The information contained in this report is copyright protected by Dental Consultants, Inc., publisher of THE DENTAL ADVISOR, and is intended for internal purposes only. Entitlement to use, reproduce or distribute this information is strictly prohibited without permission from Dental Consultants, Inc. Any use, reproduction or distribution without permission would be a copyright infringement. Copyright ©2014 – All rights reserved.



